Disclaimer: This study is provided for educational purposes only. The U.S. Pain Foundation does not endorse or recommend the use of any particular product, medication, remedy, procedure or therapy. Always seek the advice of your physician or another qualified health provider with any questions you may have regarding your medical condition and appropriate treatment.
By Sree Koneru, Ph.D., Kenneth J. McLeod, Ph.D., Richard Staelin, Ph.D.
What is Chronic Pain?
Acute or short-acting pain serves as a protective mechanism, where the brain communicates to an individual that tissues in the body (skin, nerves, muscles, joints, or internal organs) are in danger of being damaged or have been damaged. Acute pain typically subsides once the damaging agent is removed, and the tissue heals. However, when pain persists far beyond normal healing times, or the pain intensity is far greater than expected for the underlying injury, it is referred to as chronic pain [1]. Chronic pain can have a musculoskeletal (pain affecting muscles, ligaments, tendons, and bones), neuropathic (pain resulting from damage to nerves), or idiopathic (pain of unknown origin) basis, but most importantly, it can significantly reduce quality of life through physical, emotional, and economic impact on the individual. Chronic pain is remarkably common, affecting 1 in 5 American adults (>50 million) with up to 1 in 12 Americans (20 million) experiencing high-impact chronic pain [2].
Chronic pain can develop when acute pain lasts for an extended period of time (days, weeks) or over shorter periods if the pain is extremely intense. Such intense pain can come from surgery or other wounds where the tissue and or nerves are cut. The development of chronic pain is associated with chemical, structural and functional changes in the central nervous system (CNS) – a process referred to as central sensitization (CS) [1]. This sensitization leads to allodynia (increased sensitivity to non-painful stimuli), hyperalgesia (enhanced pain response to painful stimuli), and temporal summation (facilitation of centrally mediated pain). CS has been reported to play a significant role in osteoarthritic knee pain, neck pain, low back pain, dysmenorrhea, fibromyalgia, myofascial pain, migraine headaches, painful bladder, among many other pain conditions [3]. To understand how acute pain transitions into chronic pain, an overview of the sensory nervous system can be helpful.
Central Sensitization – the transition from Acute to Chronic Pain
The afferent division of the body’s peripheral nervous system (in contrast to the central nervous system) translates somatic (internal) and external stimuli into a pattern of neural impulses which are transmitted to the CNS. Figure 1 illustrates the various types of afferent nerves, and the type of sensory information they transmit. Two main nerve types, which normally transmit the injury information which is interpreted as pain, are the A-delta and C fibers [4]. Following a brief damaging stimulus to a tissue, the signals carried by the A-delta and C fibers subside. However, if the damage is severe, leading to an extremely intense pain sensation, or is sustained for an extended period, the pain pathways can be fundamentally altered. This alteration occurs at the spinal cord level where biochemical changes along the nerve pathways make the nervous system hyperreactive to sensory stimuli, i.e., central sensitization (CS).
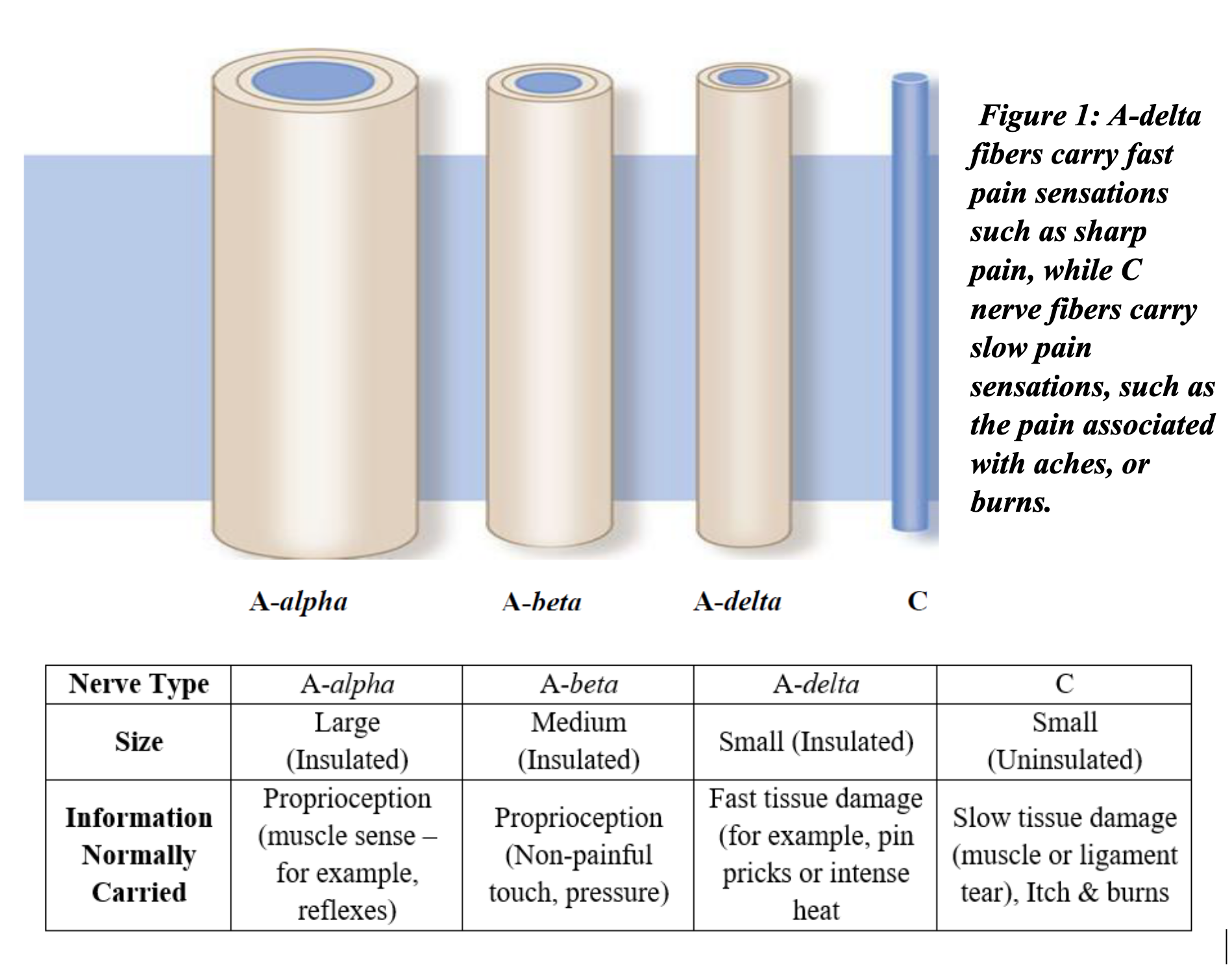
This adaptation of the nervous system results in the lowering of the individual’s threshold for stimuli that are registered as pain. The results of this change are similar to what happens when motion sensors in a home burglar alarm system are aimed too low, so that non-threating signals, such as the movements of pets, are able to set off false alarms. The system is not malfunctioning, but the threshold settings are no longer correct.
What does this central sensitization process mean when thinking about getting relief from chronic pain? First, since CS is associated with changes in the CNS it is difficult to address through pharmacotherapy without causing significant side effects. This is why common “over the counter” therapies such as non-steroidal anti-inflammatories (e.g., Ibuprofen), topical ointments, heat wraps etc. have very limited effectiveness in treating chronic pain [6]. Their effects are directed towards reducing inflammation alone and do not address the changes which have occurred in the CNS (which produces the signals which are interpreted by the brain as pain). While more powerful drugs such as opioids can block pain signals from reaching the brain, they also do not directly address CS, and this treatment modality can have very serious side effects. Due to the inability of acute pain therapies to adequately address chronic pain, the most common forms of treatment for CS involves behavioral modifications, often supplemented with antidepressants and/or anticonvulsants.
Second, an understanding of the role CS plays in chronic pain helps researchers develop new drug-free approaches for achieving pain relief. These approaches address the CNS and the pain signals that go to the brain by modulating peripheral nervous system activity, in a process referred to as neuromodulation. As discussed in more detail below, one of these neuromodulation approaches provides relief through sustained, sub-threshold (i.e. no sensation), stimulation to the painful area [7]. Consequently, when used as part of a multimodal treatment regimen, long duration neuromodulation of peripheral nerves can serve as an effective intervention for reducing chronic pain.
Electroceuticals and Neuromodulation
Therapeutic neuromodulation is defined as “the alteration of nerve activity through the delivery of a stimulus to targeted sites of the body” [8]. Targeted neuromodulation can be achieved through several methods: 1) invasive (implanted electrodes, such as with pacemakers or spinal cord stimulators); 2) semi-invasive (surface electrodes, like TENS); and 3) non-invasively using electromagnetic fields (such as with transcranial magnetic stimulation or pulsed shortwave therapy). Collectively, these neuromodulation technologies are referred to as electroceuticals in the medical device industry. We focus our attention on this third approach for several reasons. Firstly, the initial two methods provide pain relief by masking the pain signal, while the third approach is aimed at relieving pain by mitigating and/or reversing the changes to the CNS. Second, non-invasive neuromodulation using electromagnetic (EMF) fields has the distinct advantage of being able to provide therapy even when the device is applied over bandages, clothing, insoles, etc. Finally, EMF neuromodulation is incapable of producing electrical “shocks” or any other uncomfortable sensations that can occur with invasive and semi-invasive neuromodulation.
One such EMF application is Pulsed Shortwave Therapy (PSWT). This therapy relies on pulsed, high-frequency, low-power EMF to achieve neuromodulation and because of its low power is able to deliver relief using wearable, continuous use devices. PSWT devices have been clinically evaluated and found to relieve chronic pain and improve functionality associated with knee osteoarthritis, plantar fasciitis, musculoskeletal (low back pain, shoulder tendonitis, etc), and non-musculoskeletal chronic pain conditions [9] [10] [11] [12], many of which are identified as syndromes of CS. The mechanism of action is believed to be based in the realm of quantum biological phenomenon, analogous to the mechanism which birds rely upon to navigate using the earth’s magnetic field (magnetoreception) [13].
Proposed PSWT Mechanism of Action
While the mechanism by which PSWT relieves chronic pain is still under investigation, we now believe there is a direct link between the pulsing EMF and the altering of the CNS. As noted earlier, lowered pain tolerance thresholds are characteristic of chronic pain, and that providing effective relief requires that these thresholds have to be raised. The key to raising pain thresholds lies in providing new information to those newly recruited A-alpha and A-beta nerve fibers that now signal pain. This counterintuitive approach can be better understood through the processes of habituation and sensitization which occur in the peripheral nervous system (the nervous system outside the central nervous system).
The peripheral nervous system constantly receives large amounts of sensory information from the environment through peripheral sensory nerves. The background level of this activity is referred to as “afferent noise”. In order to send only relevant information to the brain, the peripheral system constantly adapts to this background noise, so that only stimuli above the background noise level are sent to the brain for processing. The raising of the background noise threshold is referred to as habituation. Conversely sensitization occurs when the background noise level threshold is lowered, i.e. the A-alpha and A-beta fibers become part of the CNS’s pain pathway.
The processes of peripheral sensitization and habituation are experienced, for example, when you move from indoors out into sunshine. When working indoors, the eyes become more sensitive to light, allowing you to see clearly in dim light. If you then move outdoors and it is a sunny day, your eyes can actually hurt from the bright light, i.e., a normal stimulus becomes painful. However, after a few seconds, your nervous system habituates and you can see clearly without pain, i.e., the system has raised the threshold defining “background” light intensity.
While peripheral sensitization is local, occurring directly in the tissue being stimulated, CS occurs in the spinal column after the injured area is exposed to sustained, or brief but intense, stimuli capable of causing pain. As discussed above this results in the A-alpha and A-beta fibers being recruited into the pain pathway. Reversal of this process requires introducing a non-painful, habituation-like stimulus to the central nervous system (i.e., an increase in the afferent noise level), directing the system to raise the pain threshold – this is the objective of PSWT.
More specifically, our working hypothesis is that this reversal process occurs when electromagnetic energy pulses increase afferent nerve activity at the site of pain thereby increasing the A-alpha and A-beta activity. In effect, the central nervous system “sees” increased afferent noise, and over time, CS is reduced through a habituation-like process, leading to a rise in the pain threshold level. Importantly, given how CS develops, PSWT can be used not only to treat chronic pain, but also to reduce pain levels in the initial phases of an injury (acute pain, postoperative pain) to reduce the risk of developing chronic pain.
Summary
A growing body of evidence demonstrates that many chronic pain conditions can be understood as syndromes of “central sensitization”, or hypersensitivity of the central nervous system. Over time, physical changes occur so that nerves which typically carry non-painful sensory information get recruited into the pain pathway, leading to lowered pain thresholds. Current pharmacological therapies have limited effectiveness as they do not address the physical changes to the central nervous system that give rise to central sensitization. Therapeutic neuromodulation via traditional electroceutical medical devices can provide acute pain relief through masking, but typically require invasive or semi-invasive use, and so treatment durations are limited. Pulsed Shortwave Therapy (PSWT) technology is a non-invasive technology which uses high-frequency electromagnetic fields (EMF) to achieve neuromodulation, without producing uncomfortable sensations during use. Often this type of treatment can be used 24/7. PSWT provides pain relief by reversing the process of central sensitization and elevating pain thresholds. Clinical research utilizing commercially available PSWT devices indicates that PSWT intervention has significant potential to both treat chronic pain, and, under acute pain conditions, to reduce the risk of developing chronic pain.
References
[1] A Feizerfan, FRCA, G Sheh, BHB MBChB FAFRM(RACP) FFPMANZCA, “ransition from acute to chronic pain,” Continuing Education in Anaesthesia Critical Care & Pain, vol. 15, no. 2, pp. 98-102, 2015.
[2] Dahlhamer J, Lucas J, Zelaya, C, et al., “Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults — United States, 2016.,” MMWR Morb Mortal Wkly Rep, pp. 1001-1006, 2018.
[3] C. J. Woolf, “Central sensitization: Implications for the diagnosis and treatment of pain,” Pain, pp. S2-15, 2011.
[4] J. Feher, “Cutaneous Sensory Systems,” in Quantitative Human Physiology, 2012, p. 326.
[5] Lluch E, Torres R, Nijs J, van Oosterwijck J., “Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review.,” Eur J Pain., vol. 18, no. 10, pp. 1367-75, 2014.
[6] Foster, N.E. et.al., “Research priorities for nonpharmacological therapies for common musculoskeletalproblems: nationally and internationally agreed recommendations,” BMC Musculoskelet Disord, vol. 10, no. 1, p. 3, 2009.
[7] Nijs, Jo et.al., “How to explain central sensitization to patients with ‘unexplained’ chronic musculoskeletal pain: Practice guidelines,” Manual Therapy, pp. 413-418, 2011.
[8] INS, “What Is Neuromodulation,” INS, 6 February 2018. [Online]. Available: https://www.neuromodulation.com/about-neuromodulation. [Accessed 13 August 2018].
[9] Bagnato, G.L. et.al., “Pulsed electromagnetic fields in knee osteoarthritis: a double blind, placebo-controlled, randomized clinical trial,” Rheumatology, pp. 3-17, 2015.
[10] Brook, J. et.al., “Pulsed radiofrequency electromagnetic field therapy: a potential novel treatment of plantar fasciitis,” J Foot Ankle Surg, pp. 312-316, 2012.
[11] I. Rawe, S. Koneru and R. Staelin, “Pulsed Shortwave Therapy in Cervical Osteoarthritis: an NSAID- Controlled, Randomized Clinical Trial,” SN Comprehensive Clinical Medicine, pp. 166-175, 2020.
[12] R. Staelin, S. Koneru and I. Rawe, “Chronic Back Pain Therapy Using the ActiPatch: A Registry of Pain Relief, Medical Use and its Side Effects,” Pain Management , vol. 7, no. 2, pp. 99-111, 2017.
[13] S. Chen, “Birds Use Quantum Mechanics to See Magnetic Fields, New Research Suggests,” Gizmodo, 23 June 2021. [Online]. Available: https://gizmodo.com/birds-use-quantum-mechanics-to-see-magnetic-fields-new-1847159559.
Author Bio
Sree Koneru, Ph.D.
 Dr. Koneru obtained his doctoral degree in Biomedical Engineering from the State University of New York at Binghamton, NY- USA. His research expertise is in investigating physiological responses of pulsed ra
Dr. Koneru obtained his doctoral degree in Biomedical Engineering from the State University of New York at Binghamton, NY- USA. His research expertise is in investigating physiological responses of pulsed ra
diofrequency electromagnetic fields for the development of advanced electroceutical devices. He leads new product development at BioElectronics, with an emphasis on prototype development and validation through pilot clinical studies.
Kenneth J. McLeod, Ph.D.
 Dr. McLeod obtained his doctoral degree in Electrical Engineering from the Massachusetts Institute of Technology (MIT), MA – USA. His research is focused on musculo-skeletal adaptation, with a particular focus on electromagnetic interactions with living tissue. He serves as Director of the Clinical Science and Engineering Research Laboratory at Binghamton University.
Dr. McLeod obtained his doctoral degree in Electrical Engineering from the Massachusetts Institute of Technology (MIT), MA – USA. His research is focused on musculo-skeletal adaptation, with a particular focus on electromagnetic interactions with living tissue. He serves as Director of the Clinical Science and Engineering Research Laboratory at Binghamton University.
Richard Staelin, Ph.D.
 Dr. Staelin is the Gregory Mario and Jeremy Mario Professor of Business Administration at the Fuqua School of Business, Duke University. He joined Duke in 1982 after teaching at Carnegie-Mellon University for 13 years. He has also been on the faculties of the Australian Graduate School of Management and the University of Chicago. He advises on the design, implementation and data analysis of clinical trials related to medical devices at BioElectronics Corporation.
Dr. Staelin is the Gregory Mario and Jeremy Mario Professor of Business Administration at the Fuqua School of Business, Duke University. He joined Duke in 1982 after teaching at Carnegie-Mellon University for 13 years. He has also been on the faculties of the Australian Graduate School of Management and the University of Chicago. He advises on the design, implementation and data analysis of clinical trials related to medical devices at BioElectronics Corporation.
Disclosure Statement
Sree Koneru is an employee of BioElectronics Corporation. Richard Staelin and Ken McLeod are consultants for BioElectronics Corporation.
