Researchers, clinicians, and advocates from around the world gathered to explore new frontiers in chronic pain management at the International Association for the Study of Pain (IASP) 2024 World Congress, which took place in Amsterdam in August. The U.S. Pain Foundation was fortunate to attend, thanks to the support of Eli Lilly and Company, and learn about promising advancements that could reshape how we understand and treat chronic pain.
Here’s what we discovered, and why there’s new hope on the horizon for those living with chronic pain.
New Approaches in Medication
Presenters explored innovative approaches to address the limitations of current treatments for moderate-to-severe pain. Research indicates that 75% of patients in the surgical setting experience acute postoperative pain, and over 50% of them report inadequate pain relief following surgery due to limitations in the safety or effectiveness of current treatment options.
The challenge in pain management has been to balance pain relief interventions to enhance patient quality of life and function while minimizing adverse reactions. For years, over-the-counter pain medication such as NSAIDs or acetaminophen have been the primary choice for pain management, but concerns persist regarding their effectiveness and safety, especially for long-term use. Opioids may be prescribed as an alternative, but they come with their own set of risks and challenges.
Both doctors and researchers emphasized the importance of gaining a better understanding of the complexities of pain biology—and the discovery of dual NOP/MOP receptor (NMR) agonists may provide a solution. In a session sponsored by Tris Pharma, attendees learned about pre-clinical data indicating that dual-NMR agonists (a substance that binds to a cell receptor to stimulate activity or produce a certain response) demonstrate a positive analgesic effect to target pain, with lower abuse potential and limited physical dependence—critical concerns for patients depending on stronger medications for chronic pain relief. This could represent a breakthrough in improving treatments across different pain conditions such as acute, burn, neuropathic, and inflammatory pain.
Another session, sponsored by Grunenthal, investigated the role of topical treatments, such as transient receptor potential vanilloid 1 (TRPV1) agonists, in managing painful diabetic peripheral neuropathy (DPN). The capsaicin 8% topical system, a TRPV1 ion channel activator that is administered through in-office visits, was highlighted for its potential to progressively reduce patients’ pain—with increased relief and treatment success observed when multiple treatments were administered. Other data indicated self-reported outcomes of improved mood and quality of life increased among patients on this topical treatment.
A Holistic Approach to Pain Research
In another enlightening session, Helene Langevin, MD, director of the National Institutes of Health’s National Center for Complementary and Integrative Health, emphasized the importance of a whole-person approach to pain. This approach extends beyond treating pain as a symptom and considers the entire person, integrating psychology, behavior, social and environmental factors, and the physical aspects of pain. Langevin stressed the need to bridge gaps in pain research, particularly by exploring the connections between cellular and molecular structures and the psychology of pain. Pain is not simply a physical sensation—it is deeply intertwined with mental health, emotional well-being, and how we experience the world.
This whole-person approach offers hope for more-effective treatment plans that can help people manage their pain in a way that supports their overall well-being. For instance, the critical interplay between impaired sleep and chronic pain was explored in another session, illustrating how untreated insomnia can exacerbate both pain and mental health conditions. The message was clear: improving sleep should be a priority in pain management strategies, as sleep disruptions have far-reaching effects on the overall well-being of individuals living with chronic pain.
Biomarkers and Their Potential for Precision Pain Management
The significant role biomarkers can play in advancing chronic pain awareness, diagnosis and treatment was also a topic of discussion. Biomarkers are measurable indicators—such as proteins, genes, molecules, or even certain behaviors, events, or social factors—that can provide insight into various biological processes. They are already playing a significant role in diagnostic, predictive, and therapeutic development for a range of diseases, and chronic pain research is now starting to catch up.
Dr. Ram Arudchandran, program director of the NIH HEAL Biomarker Program, discussed seven categories of biomarkers, including diagnostic, predictive, pharmacodynamic, and susceptibility/risk markers, all of which could revolutionize pain management by increasing the success rate of developing new therapies. For example, NIH HEAL researchers are now investigating biomarkers to identify the persistence of post-traumatic headache, or to predict which patients with breast cancer might be more likely to also develop peripheral neuropathy. Additionally, if researchers can determine specific pharmacodynamic biomarkers, clinicians may be able to offer more targeted and effective therapies in the future.
Other biomarker-related research is exploring how to better identify whether a person’s pain is nociceptive, nociplastic, or neuropathic, and the differences between those types of pain. While understanding these distinctions may not always tell clinicians exactly what’s causing a person’s pain, it can aid in tailoring treatments to better suit individuals’ needs and conditions.
However, the field still faces challenges around inconsistent terminology, with different researchers and clinicians using non-standardized terms related to pain. Reconciling these differences could bring us closer to more precise, targeted treatments that better meet the needs of individual patients.
Transforming Clinical Trials with a Chronic Pain Master Protocol
One intriguing discussion centered around the chronic pain master protocol (CPMP), a clinical trial design developed by Eli Lilly and Company that could transform how new pain treatments are developed. Currently, the probability of FDA approval for pain therapeutics is a dismal 0.7%, compared to 6.5% for all other diseases.
The protocol allows researchers to test multiple drugs across different pain populations in a single trial —currently, the trial is exploring treatment options simultaneously for patients with osteoarthritis, chronic low back pain, and diabetic peripheral neuropathy. This innovative design aims to speed up clinical drug development, improve trial efficiency, and bring medicines to patients more quickly by assessing the safety and efficacy of multiple novel chronic pain medicines at once. This process would help refine phenotyping (analyzing patients’ characteristics caused by genes or environmental factors) to pinpoint which patients are most likely to benefit from specific therapies.
The session emphasized the need for clinical trials to move beyond traditional models. For instance, through the CPMP’s unique model, over 4,500 individuals were screened for 12 separate studies, leading to the identification of four novel pain therapeutics for study since the CPMP’s inception in mid-2020.
Because of the multi-disease aspect of this protocol, researchers have a greater opportunity to re-assess if a particular molecule that didn’t work for one disease state could have some application for a different type of pain. Researchers expressed plans to remain at the forefront of pain research and utilize this protocol in other studies and for other pain conditions.
Phenotyping for Precision Medicine
The future of precision medicine in pain management was also covered. Precision medicine tailors pain treatments to the unique characteristics of each patient, optimizing treatment efficacy and minimizing unnecessary side effects. Researchers are creating a database of phenotypic and molecular data to guide future clinical trials. Eli Lilly and Company’s CPMP has been successful in gathering high-quality data through platform trials, allowing for the examination of millions of data points across different chronic pain conditions. This approach ensures a strong evidence base for treatments moving into the clinical phase.
Another topic that prompted discussion between researchers and advocates was the “pain catastrophizing” (PCat) scale (also called PCS), commonly used in research to determine how successful a treatment may be for a certain patient. One area of research is exploring the possibility that a high pain catastrophizing “score” could serve as a biomarker for susceptibility to chronic pain.
Patient advocates suggested reflecting on the use of some biomarker terms, such as “PCat” (pain catastrophizing). From a patient perspective, this term can be dismissive, stigmatizing, and demoralizing. Advocates urged collaboration between scientists, clinicians, and patient advocacy organizations to consider changes in terminology to ensure the patient experience is validated, respected, and understood.
Expanding Pain Policy on a Global Level
Attendees discussed global efforts to bring pain management to the forefront of health agendas, with a focus on the impact of the World Health Organization’s universal health coverage resolution and the adoption of the International Classification of Diseases 11th Revision (ICD-11) on pain diagnosis and management worldwide.
Advocates emphasized the importance of leveraging media coverage and networking with diverse groups (such as pain organizations, groups focused on women, disability rights groups, and federal agencies) to influence policy, with a call for persistent advocacy.
The Patient Perspective in Research
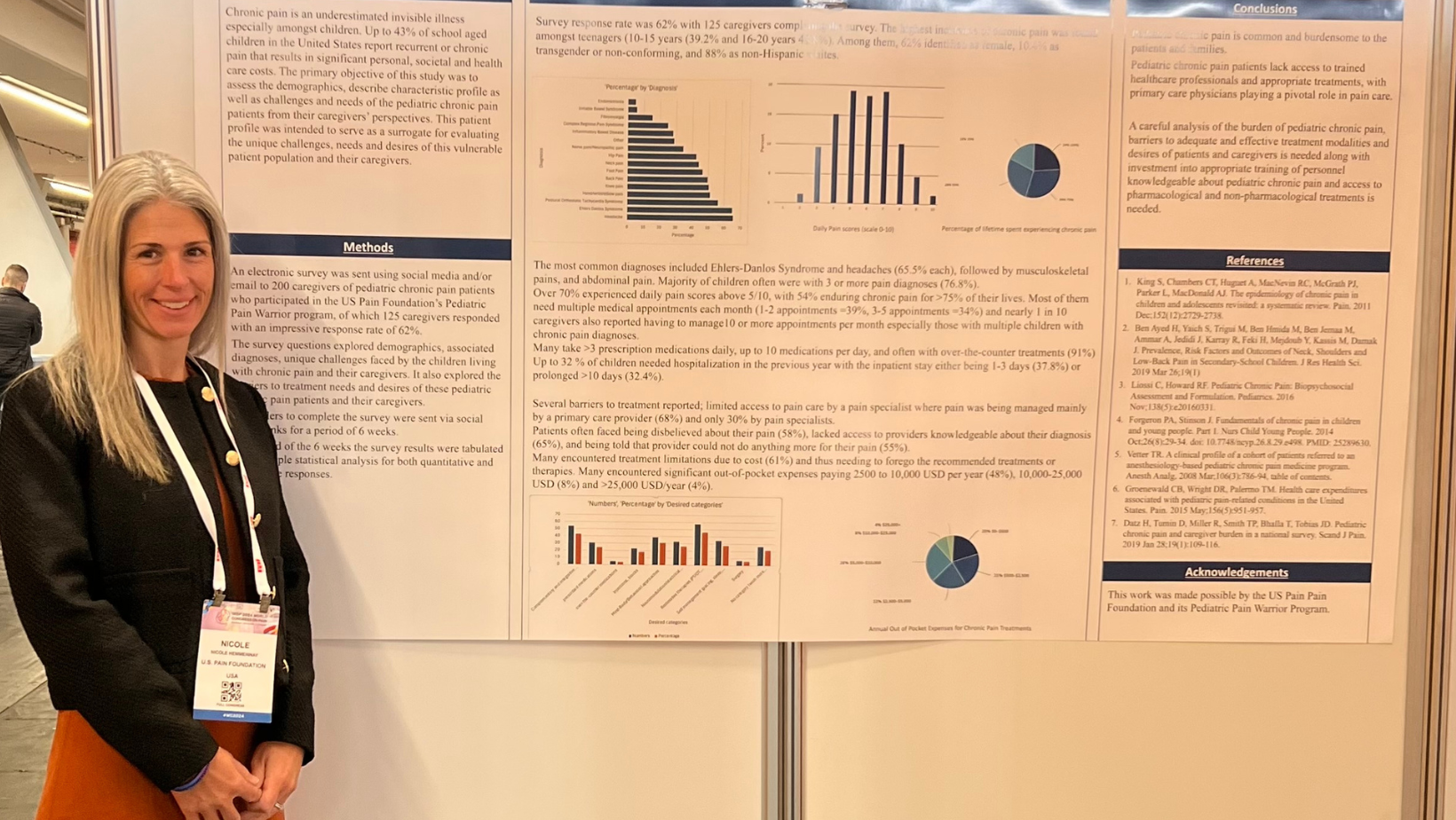
The incorporation of the patient perspective in this year’s IASP World Congress, particularly through poster presentations, represents a significant advancement in understanding and treating chronic pain. U.S. Pain was honored to be a co-author of an approved poster focused on pediatric pain (authored by Anjana Kundu, MBBS, MD, ABOIM, anesthesiologist-in-chief at University of Rochester’s Golisano Children’s Hospital and a board-certified pediatric anesthesiologist, pediatric pain, and palliative care physician). This study, based on feedback from caregivers of children living with chronic pain who are involved in U.S. Pain’s Pediatric Pain Warrior Program, shed light on the considerable personal, societal, and health care challenges faced by these young patients. Key findings revealed high daily pain scores, multiple diagnoses, and significant barriers to treatment—highlighting the need for specialized care and multidisciplinary options, as well as greater access to knowledgeable providers.
While the acceptance of this poster at the World Congress is an important step in integrating the patient perspective, there is still a need for broader representation of patient voices at research and medical conferences in the future. Without a stronger presence of patient experiences and feedback, progress in addressing the complex and multifaceted nature of chronic pain will remain incomplete.
The Path Forward
The 2024 IASP World Congress demonstrated that we are on the brink of a new era in chronic pain research. From innovative pharmacotherapies and whole-person approaches to cutting-edge biomarker research and master protocol clinical trials, there is real promise for the future. The next phase of research aims not just to treat chronic pain more effectively, but to do so in a way that acknowledges and integrates the patient’s experience.
Allan Basbaum, PhD, professor and chair of the Department of Anatomy at the University of California, San Francisco, emphasized a message that truly captured the efforts of IASP. He suggested that it may be time to add a “T” to IASP: IASTP — International Association for the Study and Treatment of Pain.
However, the journey is far from over. The research presented at the World Congress also highlighted the challenges that remain, from the need for more standardized terminology to the importance of involving diverse patient populations in clinical trials. Looking ahead, it is evident that the patient voice must guide and inform every step of the research process. Chronic pain is a deeply personal experience, and the perspectives of those who live with it every day are invaluable in shaping research priorities and treatment approaches.
For individuals living with chronic pain, these advancements offer hope. While there is still much work to be done, the future of pain management appears brighter, more personalized, and more patient-centered than ever before.
For further information about the IASP 2024 World Congress and the sessions discussed in this article, please visit iasp-pain.org.
Subscribe to our newsletter
U.S. Pain Foundation is a 501 (c)(3) nonprofit organization dedicated to serving those who live with conditions that cause chronic pain, as well as their caregivers and care providers. Learn more.
Our Sponsors
U.S. Pain Foundation relies on the generosity of donations and grants. We are especially thankful to our Corporate Council for sustaining our programs and services year-round. Learn more.
Contact Us
U.S. Pain Foundation, Inc.
15 North Main Street, Unit 100
West Hartford, CT 06107
Telephone: 800.910.2462
Email:
contact@uspainfoundation.org
Tax ID number: 26-2703521
All Content Copyright 2021 | All rights reserved. U.S. Pain Foundation is a qualified 501(c)(3) tax-exempt organization. Disclaimer
